|
|
|
Cancer-associated PTEN : Structural and Functional Characterization
|
|
|
|
|
|
|
|
|
|
|
|
|
Noushin Nabavi
|
|
|
|
University of California, San Francisco
|
Department of Cellular and Molecular Pharmacology, UCSF, 600 16th Street, MC 2140, CA 94158-2140, USA
|
|
noushin.nabavi@ucsf.edu
|
|
|
|
|
|
|
|
|
|

|
|
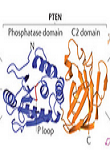
Phosphatase and tensin homolog detected on chromosome ten (PTEN) is a tumor suppressor gene protecting cells from developing cancer and forming tumors (Leslie and den Hertog). In addition, PTEN mediates cell cycle arrest, adhesion, migration, and apoptosis(Sun, Lesche et al. 1999). The complete loss or mutations in the PTEN gene has been implicated in many human cancers from mammary carcinoma to developmental abnormalities and autism. It is in fact the most common mutation in human cancers and are inherited in an autosomal dominant manner (Yamada and Araki 2001). In their recent Cell paper, Papa et al (Papa, Wan et al. 2014) shows a novel homodimerization characteristic of PTEN that renders it active. Mechanistically, the homodimerized active conformation exerts its phosphatase activity on downstream targets such as AKT, PIP3, and PI3K and decreases their oncogenic activity. The two mutated PTEN isoforms characterized in this study have a reversed functionality compared to wild-type protein. These findings have great implications in developing drug agents that target PI3K pathway and sensitive to PTEN mutations.
|
|
|
|
|
|
|
|

|
|