|
|
|
EDITORIAL: Glycolysis as a target for cancer therapy
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tuhin Subhra Chakraborty, PhD
|
|
|
|
University of Michigan
|
|
Molecular and Integrative Physiology, University of Michigan, MI 48109, USA
|
|
tchakrab@umich.edu
|
|
|
|
|
|
|
|
|
|

|
|
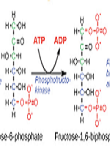
Cancer is a highly heterogeneous disease and each cancer has its individual metabolic fingerprint. Even within a single cancer, its constituent cells are heterogeneous and the metabolic fingerprint varies from one cell to another (Jie Zheng 2012). Unlike normal cells, glycolysis is enhanced in cancer cells (Warburg 1927, 1956; Robert A. Gatenby et.al. 2004; Jie Zheng 2012). This was first described by German scientist Otto Warburg in the 1920s. Following Warburg’s observations that cancer cells have a higher rate of glycolysis than normal cells, interest in the metabolic property of cancers has steadily increased. In recent years, understanding the features and complexity of the metabolism and energetics of cancer cells has been rekindled, mainly because therapy targeting metabolism hits the “core” of the cancer and has the potential to cripple a cancer cell’s ability to self-renew.
|
|
|
|
|
|
|
|

|
|