|
|
Author(s)
Martin Michael Dcona and Emil Iqbal
Address
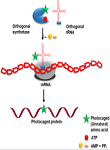
Department of Internal Medicine, Department of Physiology and Biophysics, and Massey Cancer Center VCU, Richmond, VA 23298 Abstract: The ability to achieve spatial and temporal control over biological processes and drug activation has led to many applications of “photocages”. By definition, photocages are chemical structures that are light-sensitive and is used to “cage” or restrict the activity of any molecule. Upon illumination of light at appropriate wavelength, the protecting group of the molecule is cleaved releasing the active entity. Many recent reports in research areas including molecular biology, neurosciences and drug delivery have demonstrated the applications of photocages. In this write-up, we provide a brief overview on commonly used photocages, ortho-Nitrobenzyl (o-NB) derivatives that are relevant to cancer-biology, and emphasize upon the usefulness and advantages of using these photosensitive molecules.  Author(s) Justin Fendos, PhD Address Department of Life Sciences, Dongseo University, Busan, South Korea 617716
Tan School of Genetics, Fudan University, Shanghai, China 508915
Abstract: In both political and business leadership, gender inequality is a topic of frequent discussion. When examining the literature, one can find a deluge of peer-reviewed articles linking gender differences to leadership. This makes a comprehensive review of the findings untenable. The present work, therefore, focuses instead on reviewing the results from two complementary methodological approaches: self-assessments and other-assessments. Self-assessments involve the administration of surveys and questionnaires to leaders, asking them to rate their own leadership preferences and self-efficacies. Other-assessments involve collecting data from subordinates, peers, and superiors. Surprisingly, both methods offer very similar conclusions about gender differences in leadership: women are generally perceived as being better leaders than men, especially for transformational purposes. 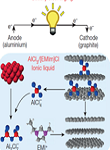
Author(s)
Haegyeom Kim, PhD
Address
Materials Sciences Division, LBNL, Berkeley, CA 94720, USA. Abstract: Graphite is actively investigated as an electrode material for rechargeable batteries because of its capability of intercalating versatile ion species combined with its low production cost and non-toxicity. This mini review will provide an overview of recent achievements as well as an outlook for graphite electrode of rechargeable batteries (i.e. Li-, Na-, K-, and Al-ion batteries), focusing on the energy storage properties of graphite electrode in various battery systems and its underlying intercalation mechanism. This mini review will provide insights into designing low-cost graphite-based energy storage systems. 
Author(s)
Theo van den Broek, M.D. Ph.D.
Address
Program in Cellular and Molecular Medicine, BCH/HMS, Boston, Massachusetts 02116, USA Abstract: Every post-doc at some point during their scientific career tried to repeat a previously published discovery but failed to replicate their findings. A survey of 1500 scientists, conducted by the journal Nature, stated that more than 70% of researchers failed in reproducing the results and more than half have failed in to reproduce their own results. This irreproducibility also has a hefty price tag, estimated at approximately $28,000,000,000 (US$28B)/year for preclinical research within life sciences alone. This replication crisis is an ongoing problem in science but new strategies to address this crisis have been initiated in the last years. 
Author(s)
Rajesh Kumar, PhD.
Address
DFCI, Boston, Massachusetts, 02140 USA Abstract: Approximately one-third of the human pregnancies have placenta-related defects including recurrent miscarriage, preeclampsia, fetal growth restriction, and uterine inflammation (Young, Levine et al. 2010, Giakoumopoulos and Golos 2013, James, Srinivasan et al. 2014). Hence it is very important to understand the pathophysiology of placental development. Pregnancy is a unique situation in which the mother and the hemi allogeneic fetus cordially coexist. Several fetal, maternal and placental mechanisms function in concert to protect the fetus from immunological recognition and rejection (PrabhuDas, Bonney et al. 2015). The placenta provides the interface for gas and nutrient exchange between the mother and the fetus and hence proper placental function is critical for healthy pregnancy. However, despite its role in sustaining pregnancy, the trophoblast stem and progenitor cells hierarchy and the underlying molecular mechanisms responsible for the development of the placenta are not well described.  Author(s) Stephanie Chrysanthou, and Myriam Hemberger Address Epigenetics Programme, Babraham Institute/Centre for Trophoblast Research, University of Cambridge, CB2 1TN, UK Abstract: The placenta is a highly specialized organ that is indispensable for intrauterine development to occur. Trophoblast cells are the major constituents of the developing placenta. They are the first cell type to arise very early in development, making up the trophectoderm, the outer layer of the blastocyst, segregating from the inner cell mass which gives rise to the embryo itself. The various functions of trophoblast cells early in development are vital for reproductive success, as they lay the foundations for a normal pregnancy and a healthy fetus. A better understanding of the mechanisms underlying these early events, including how the early trophoblast niche is regulated by transcription factors and specific epigenetic modifiers, is critical for understanding and eventually treating placental pathologies, which can inevitably cause pregnancy complications. 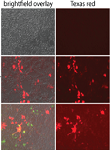
Author(s)
Tyler Ford, PhD.
Address
Cambridge, MA 02139, USA Abstract: Reproducibility, here defined as the ability to apply published research findings to future work, is key to the successful and efficient advancement of science. There are many tools available to help researchers make their work reproducible. These tools enable users to think carefully about experimental designs, to explicitly and tenaciously describe experimental procedures, and to easily/effectively share the results of their work. Addgene provides many such tools to help make research more reproducible. Indeed, Addgene’s primary role in reproducibility is to make it easier for researchers to share reagents, but Addgene also interacts with many other aspects of reproducibility in important and subtle ways. Addgene validates reagents, provides access to resources that make it easier to use reagents, and enables researchers to quickly find the proper tools for their work so they can spend more time thinking about experimental design. 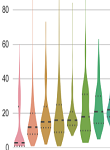
Author(s)
Yike Li, and David Zealer.
Address
Department of Otolaryngology, VU Medical Center, Nashville, Tennessee, 37232, U.S.A. Abstract: The primary objective of this study was to investigate publication delays in scholarly peerâ€reviewed journals in the field of otolaryngology, audiology and speech pathology. A list of 58 journals indexed in the Journal Citation Report by Thomson Reuters were included. Bibliographic content of publications in these journals from 2007-2017 was extracted from the National Center for Biotechnology Information databases using the “RISMed” package of R. The acceptance delay was defined as the time lapse between the submission date and the acceptance date. The editorial delay was the time lapse between the acceptance date and the publication date. These data were plotted using the “ggplot” function. A total of 28084 articles were eligible for data analysis. 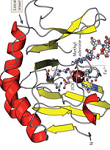
Author(s)
Rita Khoueiry, and Kian Peng Koh.
Address
KU Leuven Department of Development and Regeneration, Stem Cell Institute Leuven, 3000 Leuven, Belgium. Abstract: In 2009, a new family of DNA modifying enzymes, the Tet-eleven translocation family was identified as 2-oxoglutarate (2-OG) and Fe(II)-dependent dioxygenases. This family of enzymes comprises three proteins TET1, TET2 and TET3 that share a carboxyl-terminal core catalytic domain consisting of a conserved cysteine-rich domain, a double stranded β-helix domain and binding sites for the cofactors Fe(II) and 2-oxoglutarate. At their amino-terminal region, TET1 and TET3 have a CXXC DNA-binding domain. Interestingly, during evolution, the segment encoding the CXXC domain of TET2 was separated from the region encoding the catalytic domain and is now encoded separately by a neighboring gene, IDAX (also called CXXC4). 
Author(s)
Iain L. O. Buxton and Scott D. Barnett
Address
Myometrial Function Laboratory at the UNR, Reno School of Medicine, Reno, Nevada 89557-0318, USA Abstract: Preterm labor leads to preterm delivery in over 50% of cases. Current treatment for preterm labor is inadequate and relies on findings in other smooth muscles. No treatment can prevent labor to allow a fetus to remain in the womb until term. Regulation of contraction/relaxation of the uterine smooth muscle is poorly understood. Recent work in our laboratory has revealed major disparity between term and preterm human uterine smooth muscle that suggests new approach to drug discovery.


|
|